Explain the Difference Between Exothermic and Endothermic Reactions
5 rows The major difference between endothermic and exothermic reactions as their names suggest. Energy is absorbed from the surrounding and goes into the reaction.

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences
The final products are stable in exothermic reactions.
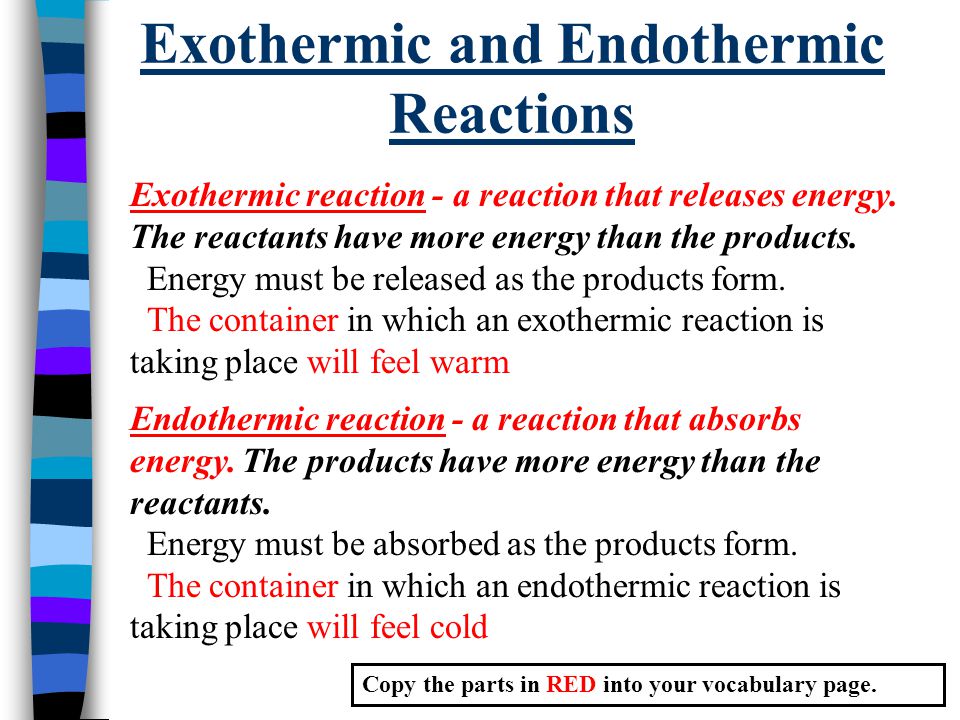
. An exothermic reaction has a negative ΔH and gives off heat to the surroundings. It can change into other forms such as heat sound light etc. The reaction made the water turn cloudy.
Let me first reveal the identity of your salts. Examples of exothermic reaction. When energy is released in an exothermic reaction the temperature of the reaction mixture increases.
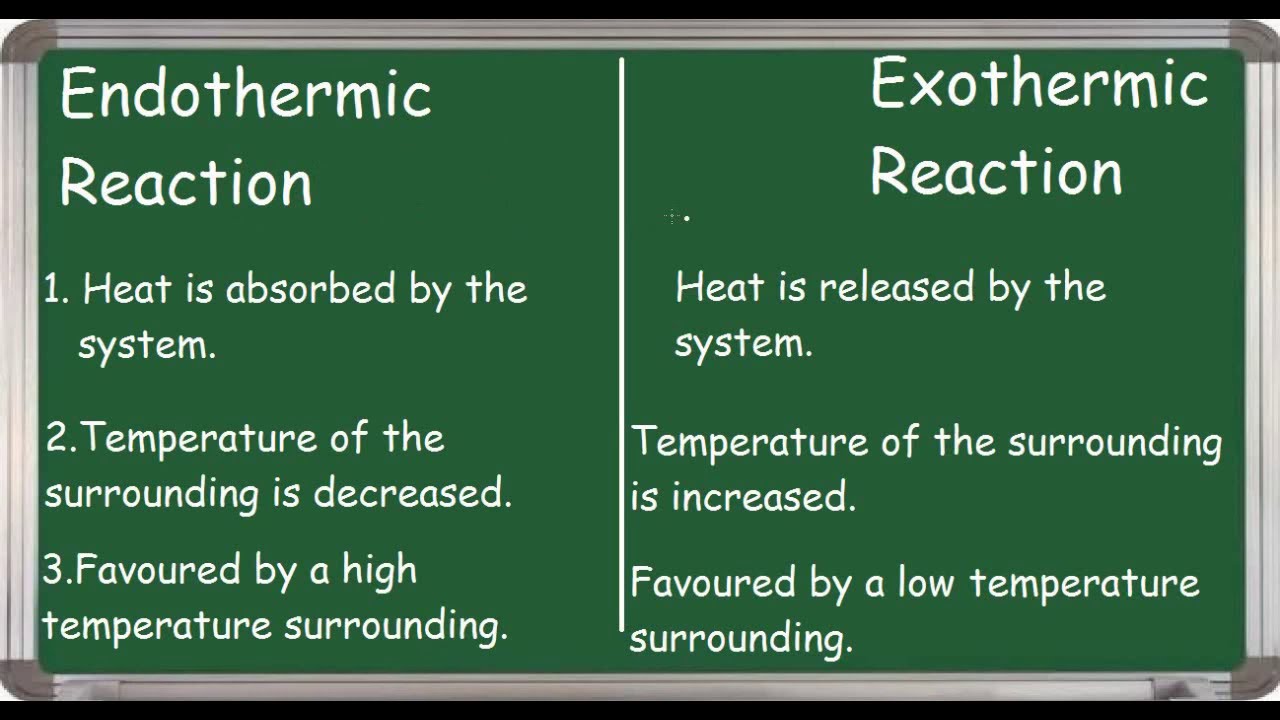
An exothermic reaction feels warm to the touch. Chemical reactions involving the use of energy at the time of dissociation to create a new chemical bond are known as endothermic reactions whereas exothermic reactions are those chemical reactions in which the energy is evolved or released. Potential energy diagrams for endothermic reactions have a positive or upward slope that represents a q or H In an endothermic reaction diagram the products end up with more stored potential energy than the reactants.
In endothermic processes reactants possess lower potential energy than the product. There are two methods for distinguishing between exothermic and endothermic reactions. Endothermic reaction Exothermic reaction Endothermic reaction is a chemical reaction which absorbs energy from the environment in the form of heat.
Endothermic reactions are chemical reactions that absorb heat energy from the. Identifying Exothermic Endothermic Reactions. Exothermic reactions are reactions or processes that releases energy usually in the form of heat or light to its environment whereas endothermic reactions are reactions that require external energy usually in the form of heat for the reaction to Proceed.
An endothermic reaction has a positive ΔH and absorbs heat from the surroundings. A good example of an endothermic reaction is photosynthesis. Exothermic reactions release energy to their surroundings because the products are lower in energy than the reactants.
The temperature decrease with the progression of endothermic reactions. During this process. In a system energy can do work.
Thats because you were given two different salts. The key difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding environment whereas exothermic reactions release energy to the surrounding environment. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding.
In contrast exothermic systems give up heat or light energy as the reaction proceeds. Difference Between Endothermic and Exothermic Reactions Definition. Salt A is ammonium nitrate and Salt B is calcium chloride.
One of your salts generated an endothermic reaction with water while the other salt generated an exothermic reaction with water. Endothermic and exothermic reactions are chemical reactions that absorb and release heat respectively. 26 Is evaporation endothermic or spontaneous.
Whats the main difference between an exothermic and endothermic reaction. The categorization of a reaction as endo- or exothermic depends on the net heat transfer. An endothermic reaction feels cold to the touch.
You can think about this visually using a reaction energy diagram as seen below. An exothermic reaction creates heat whereas a endothermic reaction absorbs heat. 12 What does one mean by exothermic and endothermic reactions explain and support your answer with one chemical equation for both.
This is due to the releasing and absorption of heat energy in the reactions respectively. An exothermic reaction releases energy into the environment. Combustion is an example of an exothermic reaction.
The endothermic reactions are when the system takes up the energy in the form of light or heat. And endothermic reaction left and an exothermic reaction right plotted on a plot of energy against the reaction coordinate a measure of the. Examples of exothermic reactions are.
Difference Between Endothermic and Exothermic Reactions. In exothermic reactions the enthalpy change is always negative while in endothermic reactions the enthalpy change is always positive. Exothermic reaction is opposite of endothermic reaction.
Reactants Products Energy. This can be used to classify. The following chemical equation can be used to describe the reaction.
This is done in the form of heat. Sara mixed water and calcium chloride. An exothermic reaction releases energy and feels warm while an endothermic reaction absorbs energy and feels cool.
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Differences between exothermic reaction and endothermic reaction. When energy is absorbed in an endothermic reaction the temperature decreases.
It releases energy in the form of heat. Photosynthesis is a popular example of an endothermic chemical reaction. An exothermic reaction has a negative enthalpy difference.
Energy is the capacity to do work. Thus in order to react they absorb the energy from the environment. The end products of endothermic reactions are less stable.
The end products are stable in exothermic reactions. Compare the slope of the line on a potential energy diagram for an endothermic reaction and an exothermic reaction. 25 What is the biggest difference between an exothermic and endothermic process.
Endothermic Vs Exothermic Reaction Differences Youtube

Difference Between Endothermic And Exothermic Reactions Chemistry

Key Question What Is The Difference Between Exothermic And Endothermic Reactions Warm Up Name 2 Ways You Could Speed Up A Chemical Reaction Ppt Video Online Download
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples
No comments for "Explain the Difference Between Exothermic and Endothermic Reactions"
Post a Comment